Indicator Titration Role . In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. Indicators are substances that change colour when they are added to acidic or alkaline solutions; This page assumes that you know. This guide will take you through the. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. The indicator is an organic dye which changes colour depending on ph. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Usually this colour change is very abrupt, taking place with the. In many titrations, you use a chemical.
from www.slideshare.net
This guide will take you through the. This page assumes that you know. Indicators are substances that change colour when they are added to acidic or alkaline solutions; Usually this colour change is very abrupt, taking place with the. In many titrations, you use a chemical. The indicator is an organic dye which changes colour depending on ph. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration.
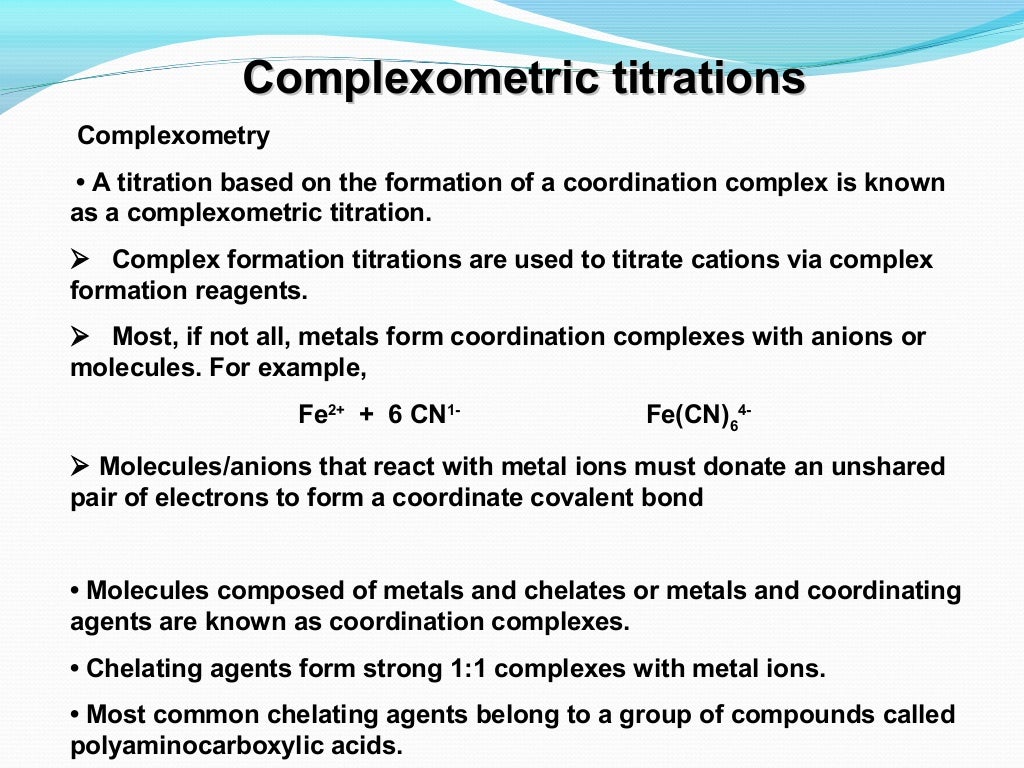
Complexometric titrations
Indicator Titration Role This page assumes that you know. This guide will take you through the. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. The indicator is an organic dye which changes colour depending on ph. Usually this colour change is very abrupt, taking place with the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. Indicators are substances that change colour when they are added to acidic or alkaline solutions; This page assumes that you know. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical.
From www.vrogue.co
Titration Indicator Types Procedure Indicators vrogue.co Indicator Titration Role This page assumes that you know. In many titrations, you use a chemical. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. Indicators are substances that change colour when. Indicator Titration Role.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Indicator Titration Role The indicator is an organic dye which changes colour depending on ph. In many titrations, you use a chemical. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known. Indicator Titration Role.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Indicator Titration Role In many titrations, you use a chemical. The indicator is an organic dye which changes colour depending on ph. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known. Indicator Titration Role.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID5572517 Indicator Titration Role The indicator is an organic dye which changes colour depending on ph. This guide will take you through the. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In many titrations, you use a chemical. Titration is an analytical technique that allows the quantitative determination of. Indicator Titration Role.
From mmerevise.co.uk
Titrations and Uncertainties MME Indicator Titration Role An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. This page assumes that you know. This guide will take you through the. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. We have stated that a good indicator should have a pkin. Indicator Titration Role.
From ceias.nau.edu
Potentiometry Indicator Titration Role This page assumes that you know. Usually this colour change is very abrupt, taking place with the. In many titrations, you use a chemical. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In a titration, you determine an unknown concentration of a sample by adding. Indicator Titration Role.
From mungfali.com
Acid Base Titration Indicator Indicator Titration Role In many titrations, you use a chemical. This guide will take you through the. This page assumes that you know. Indicators are substances that change colour when they are added to acidic or alkaline solutions; An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. Titration is an analytical technique that allows the. Indicator Titration Role.
From www.numerade.com
SOLVED Using the table of indicators identify which of the given Indicator Titration Role In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. The indicator is an organic dye which changes colour depending on ph. This page assumes that you know. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point.. Indicator Titration Role.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Indicator Titration Role Indicators are substances that change colour when they are added to acidic or alkaline solutions; In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by. Indicator Titration Role.
From www.youtube.com
Role of indicator and titration experiment YouTube Indicator Titration Role This page assumes that you know. In many titrations, you use a chemical. Usually this colour change is very abrupt, taking place with the. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. We have stated that a good indicator should have a pkin value that is close to the expected ph. Indicator Titration Role.
From www.slideshare.net
Complexometric titrations Indicator Titration Role Usually this colour change is very abrupt, taking place with the. The indicator is an organic dye which changes colour depending on ph. This page assumes that you know. Indicators are substances that change colour when they are added to acidic or alkaline solutions; In a titration, you determine an unknown concentration of a sample by adding a second reactant. Indicator Titration Role.
From thechemistrynotes.com
Titrimetric Analysis Terms, Types, Principle, Advantages Indicator Titration Role This guide will take you through the. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. This page assumes that you know. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. Indicators are substances that change. Indicator Titration Role.
From www.vrogue.co
Acid Base Titration Setup Phenolphthalein Indicator V vrogue.co Indicator Titration Role In many titrations, you use a chemical. The indicator is an organic dye which changes colour depending on ph. An indicator in titration is a substance that changes its color when the reaction reaches its endpoint. Usually this colour change is very abrupt, taking place with the. This guide will take you through the. This page assumes that you know.. Indicator Titration Role.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Indicator Titration Role We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. This page assumes that you know. Usually this colour change is very abrupt, taking place with the. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition. Indicator Titration Role.
From www.numerade.com
SOLVEDa. What is meant by the end point of a titration? b. What is the Indicator Titration Role This guide will take you through the. Indicators are substances that change colour when they are added to acidic or alkaline solutions; We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. An indicator in titration is a substance that changes its color when the reaction reaches. Indicator Titration Role.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce Indicator Titration Role We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. Titration is an analytical technique that allows the quantitative determination of a specific substance dissolved in a sample by addition of a reagent. In a titration, you determine an unknown concentration of a sample by adding a. Indicator Titration Role.
From studylib.net
The titration of an Indicator (HIn) Indicator Titration Role We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. In many titrations, you use a chemical. Indicators are substances that change colour when they are added to. Indicator Titration Role.
From psu.pb.unizin.org
14.7 AcidBase Titrations (2018) Chemistry 112 Chapters 1217 of Indicator Titration Role The indicator is an organic dye which changes colour depending on ph. In a titration, you determine an unknown concentration of a sample by adding a second reactant of known concentration. We have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In many titrations, you use a. Indicator Titration Role.